Centre Issues Advisory on Paediatric Cough Syrups: The Indian Ministry of Health and Family Welfare has issued an urgent advisory to all states and Union Territories following the tragic deaths of children in Madhya Pradesh and Rajasthan, raising concerns about the use of paediatric cough syrups. While initial reports suggested a possible link between the consumption of certain syrups and kidney failure, laboratory analyses have found no evidence of toxic contaminants such as diethylene glycol or ethylene glycol, which are known to cause kidney damage.
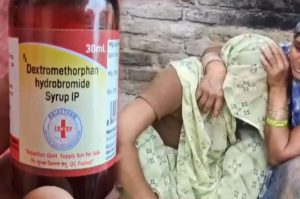
The government’s advisory focuses on the rational use of cough syrups in children, underlining the need for careful prescription, dosage adherence, and vigilance by healthcare providers to prevent any potential adverse effects.
Centre Issues Advisory on Paediatric Cough Syrups: Tragic Incidents in MP and Rajasthan
Between late September and early October 2025, several cases of acute kidney complications were reported among children in Chhindwara district, Madhya Pradesh, and Sikar and Bharatpur districts in Rajasthan.
- In Chhindwara, six children reportedly succumbed to severe kidney-related illnesses within a span of two weeks.
- Following these events, two more child deaths were reported in Rajasthan under similar circumstances.

Early investigations had suggested a possible link to a commonly prescribed cough syrup used for children. The health departments in both states initiated probes, suspended prescribers, and banned the sale of the suspected syrups in affected districts while maintaining public alerts for vigilance.
These incidents triggered national attention, prompting the Director General of Health Services (DGHS) and the Central Drugs Standard Control Organisation (CDSCO) to coordinate testing and issue safety advisories.
Government Advisory: Promoting Safe Use of Cough Syrups
The Ministry of Health and Family Welfare has issued a detailed advisory highlighting the following measures for all states and Union Territories:
- Ensure rational prescription and usage of cough syrups in children.
- Strengthen adverse drug reaction monitoring systems at hospitals and clinics.
- Raise awareness among healthcare providers about safe administration and dosage of medicines in pediatric populations.
- Conduct quality control and batch verification to prevent the circulation of counterfeit or substandard medications.
- Advise parents to avoid self-medication and consult pediatricians before administering any cough syrup.
The advisory aims to reassure the public that no toxic substances were detected while emphasizing caution in medication practices for children.
For official government guidelines on pediatric medicines, visit the Ministry of Health and Family Welfare and CDSCO.
Laboratory Findings: No Toxic Contaminants Detected
Samples of the suspected cough syrups were collected and sent to several premier institutions for testing, including:
- National Centre for Disease Control (NCDC)
- National Institute of Virology (NIV)
- Central Drugs Standard Control Organisation (CDSCO)


Tests confirmed that the syrups did not contain diethylene glycol, ethylene glycol, or any other nephrotoxic chemicals. While this is a relief, health experts caution that further studies are needed to determine whether other ingredients, underlying health conditions, or storage conditions could have contributed to the tragic outcomes.
The government has stressed the importance of continuous pharmacovigilance, ensuring that all pediatric formulations meet safety standards before reaching the public.
Public Health Implications
These events highlight multiple critical issues in India’s healthcare landscape:
- Safety of Pediatric Medicines: Children are highly susceptible to adverse drug reactions. Stringent regulation, quality control, and monitoring are vital to prevent harm.
- Healthcare Infrastructure in Rural Areas: Access to quality healthcare and trained medical professionals is limited in many districts, which can exacerbate health risks.
- Pharmacovigilance Systems: Effective monitoring of adverse drug events and swift government response are essential to safeguard children.
- Awareness Among Caregivers: Parents must be educated on proper dosages, potential side effects, and the importance of consulting qualified pediatricians before administering medications.
The incidents serve as a reminder of the need for a robust drug safety ecosystem encompassing manufacturers, regulators, doctors, and caregivers.
Expert Opinions
Pediatricians and pharmacologists have weighed in on the situation:
- Dr. Ramesh Kumar, senior pediatrician in New Delhi, noted: “The absence of known toxic contaminants in the cough syrup is reassuring, but these cases highlight the need for meticulous monitoring of all medications administered to children. Even over-the-counter medicines can have risks if used improperly.”
- Dr. Anjali Mehta, pharmacologist, added: “Storage conditions, expiry dates, and potential interactions with other medications can sometimes contribute to adverse outcomes. Parents and practitioners must remain vigilant.”
These insights underline that safe medicine use involves more than just ingredient checks — it includes prescription practices, parental awareness, and proper storage.
Lessons Learned and Preventive Measures
- Regulatory Oversight: CDSCO and state health departments are urged to enhance inspection protocols for pediatric drug manufacturing facilities.
- Training Healthcare Providers: Doctors and pharmacists should be updated on adverse reaction monitoring, dosage guidelines, and potential risk factors.
- Public Awareness Campaigns: Campaigns on safe pediatric medication practices can help prevent accidental misuse or overuse.
- Research and Investigation: Ongoing research is necessary to identify other potential causes of kidney failure and ensure transparency in investigations.
The Ministry of Health has pledged to review current systems and strengthen pediatric drug safety mechanisms across the country.
Conclusion
While laboratory tests have ruled out the presence of nephrotoxic chemicals in the cough syrups linked to child deaths, the incidents in Madhya Pradesh and Rajasthan underscore the importance of vigilance, rational medicine use, and strong regulatory oversight.
The government advisory serves as a timely reminder for healthcare providers, parents, and authorities to collaborate in safeguarding children’s health. Continued monitoring, awareness campaigns, and strict adherence to pediatric medication guidelines are crucial to prevent future tragedies.
The Indian health authorities emphasize that no cough syrup should be administered to children without proper consultation, and parents are advised to follow dosing instructions carefully, store medicines properly, and report any adverse reactions promptly.
External References
- Ministry of Health and Family Welfare – Official Portal
- Central Drugs Standard Control Organisation – CDSCO
- National Centre for Disease Control – NCDC
- National Institute of Virology – NIV
- Pharmacovigilance Programme of India
Also read: Home | Channel 6 Network – Latest News, Breaking Updates: Politics, Business, Tech & More

