Madhya Pradesh – wIn a major development, the WHO Cough Syrup Warning has sent shockwaves through India’s pharmaceutical sector. The World Health Organisation (WHO) issued a serious alert identifying three cough syrups linked to the deaths of 22 children in Madhya Pradesh. The syrups—Coldrif, Respifresh TR, and ReLife—have been found to contain highly toxic levels of diethylene glycol (DEG), a lethal industrial chemical.
This WHO Cough Syrup Warning follows weeks of investigation into the tragic deaths of children in Parasia village, Chhindwara district, allegedly caused by contaminated medicines. Health authorities are now under pressure to ensure no such products are circulating in the market.
Toxic Coldrif Syrup at the Centre of WHO Cough Syrup Warning
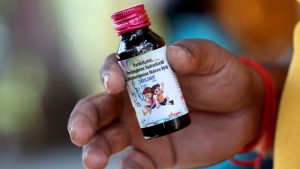
The WHO Cough Syrup Warning specifically names Coldrif syrup, produced by Tamil Nadu-based Sresan Pharmaceuticals, as one of the contaminated products. Laboratory tests confirmed that Coldrif contained DEG nearly 500 times above the permissible limit. The syrup, consumed by several children under the age of five, triggered a severe poisoning outbreak that claimed multiple lives in Madhya Pradesh.
In response to the WHO Cough Syrup Warning, the Central Drugs Standard Control Organization (CDSCO) initiated a nationwide inspection of pharmaceutical units. Sresan Pharmaceuticals’ license was fully revoked, and its owner, G. Ranganathan, was arrested for manufacturing and distributing the toxic syrup.
WHO Identifies Three Deadly Syrups in Its Warning

The WHO Cough Syrup Warning lists three Indian-made products—Coldrif from Sresan Pharmaceuticals, Respifresh TR from Rednex Pharmaceuticals, and ReLife from Shape Pharma. According to the global health agency, all three syrups pose a significant health risk and can lead to life-threatening illnesses.
WHO officials stated that the syrups could cause serious damage to the kidneys, liver, and nervous system if consumed. The agency has urged health authorities worldwide to immediately report if any of the identified medicines are detected in their countries. This global call reinforces the gravity of the WHO Cough Syrup Warning.
Indian Authorities Respond to WHO Cough Syrup Warning
Following the WHO Cough Syrup Warning, India’s CDSCO confirmed that the toxic syrups contained dangerously high concentrations of DEG—up to 48%—far above the safe limit of 0.1%. The CDSCO has assured the WHO that none of these contaminated products were exported. This clarification aims to prevent panic among importing nations.
The WHO Cough Syrup Warning comes amid growing scrutiny of India’s pharmaceutical exports. The country had faced similar controversies in previous years over unsafe medicines linked to fatalities abroad. This latest alert has reignited debates over manufacturing oversight and quality assurance in India’s drug industry.
Government Crackdown and Safety Measures
In the aftermath of the WHO Cough Syrup Warning, the Indian government issued an advisory to all states and union territories. It directed medical professionals to exercise extreme caution when prescribing cough syrups to children, especially those under two years old. The advisory emphasized that cough syrups are not generally recommended for children below five years of age.
The WHO Cough Syrup Warning prompted a series of raids and inspections across Tamil Nadu, where several pharmaceutical plants came under the scanner. Health officials discovered inadequate manufacturing practices, poor quality control, and non-compliance with safety norms in certain facilities.
Impact of WHO Cough Syrup Warning on India’s Pharma Industry

The WHO Cough Syrup Warning has cast a shadow on India’s reputation as the “pharmacy of the world.” With over 60% of vaccines and a large share of generic medicines supplied globally from India, the incident has raised international concerns about regulatory enforcement. Industry experts warn that rebuilding confidence will require strict compliance, transparency, and regular audits.
The WHO Cough Syrup Warning also underscores the urgent need for better traceability systems and digital tracking of pharmaceutical production. Public health experts believe that stronger regulation can prevent tragedies like the Madhya Pradesh incident.
Ensuring Accountability After WHO Cough Syrup Warning
The WHO Cough Syrup Warning serves as a wake-up call for both national and state-level health authorities. Sresan Pharmaceuticals’ manufacturing failures have exposed gaps in the drug approval and monitoring process. Authorities have now ordered detailed safety audits of all cough syrup manufacturing units.
As part of follow-up action, the WHO Cough Syrup Warning has also prompted India’s health regulators to collaborate with the global agency to improve data sharing and product testing protocols. This cooperation is seen as crucial for rebuilding international trust in Indian pharmaceuticals.
A Critical Turning Point for Drug Safety
The WHO Cough Syrup Warning marks a crucial moment in India’s public health system. The tragedy that led to the loss of young lives has become a powerful reminder of the consequences of regulatory neglect. Moving forward, experts stress that the health and safety of children must remain the top priority.
As the government tightens surveillance and WHO continues to monitor the situation, the WHO Cough Syrup Warning stands as a global call for stronger vigilance, transparency, and accountability across the pharmaceutical world.

